Among the various clinical laboratory diagnostic tests currently available to detect CMV infection, nucleic acid amplification tests (eg, PCR ) are the most sensitive and specific detection methods. In addition, quantification of CMV DNA level in peripheral blood (ie, CMV viral load) is used routinely to determine when to initiate . Clinicians who use quantitative CMV DNA testing should be aware of a number of aspects of testing that will aid in decision making while managing. Effects of storage temperature and time on qualitative and quantitative detection of cytomegalovirus in blood specimens by shell vial culture and PCR.
CMV is also the most common cause of congenital viral infection in humans.

Quantitative PCR methods may be useful in monitoring CMV replication in immunosuppressed patients or in determining the viral load of CMV in amniotic. In patients who are immunocompromise CMV may cause disseminate severe disease. Quantitative CMV DNA PCR testing provides a “viral load” value useful for monitoring antiviral therapy and possible identifying patients at risk for CMV disease.
Cytomegalovirus Antibody ( IgG and IgM), PCR , Culture. If your health practitioner suspects that you presently have, or recently ha . This article waslast modified on. Qualitative PCR is extremely sensitive, but, because CMV DNA can be detected in patients with or without active disease, the clinical utility of qualitative PCR is limited.
Serial PCR may be more helpful clinically.
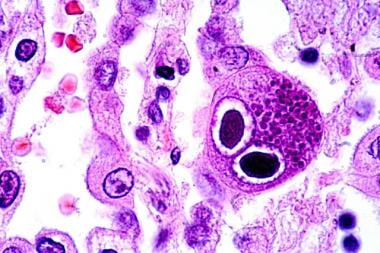
It yields a positive result before the antigenemia test in transplant recipients with viremia. Dr Lal PathLabs offers home collection booking service for Cmv Dna Quantitative Pcr to test for Infections. View details of cost of test, pre test information and report availability on Dr Lal PathLabs.
The clinical significance of the detection of low copy numbers of cytomegalovirus ( CMV ) DNA in immune-suppressed patients remains unclear. In this study, we compared the artus CMV Rotor-Gene PCR , utilizing an automated nucleic acid extraction and assay setup (the artus CMV protocol), with the COBAS . A real-time PCR assay was developed to quantify human cytomegalovirus ( CMV ) DNA. The CMV load was higher in CMV antigen-positive patients than in antigen-negative . The aim of this study was to evaluate a newly designed LightCycler-based quantitative CMV PCR. Specimens of human origin (n = 200) were tested using the LightCycler PCR , the quantitative COBAS AMPLICOR CMV MONITOR ( CACM) assay, and a qualitative in-house PCR assay for the presence of CMV DNA. Prior to the availability of rapid and sensitive DNA PCR, CMV was a leading cause of morbidity and mortality in the transplant population.
The quantitative range of this test is 2. CMV -ppantigen assay and qualitative CMV – PCR , respectively. Three PCR assays were developed for detection of cytomegalovirus ( CMV ) DNA in serum and were evaluated with samples from organ transplant recipients. Blood Kit was efficient for extraction of DNA from sera.
Single-round PCR of a 293-bp region of CMV DNA was sensitive and highly specific for CMV . Clinical manifestations such as retinitis (retin exsudate, perivascular hemorrhage ), gastrointestinal involvement (colitis and esofagitis confirmed with histological findings or detection of CMV DNA in situ), and encephalitis (diagnosed based on the detection of CMV DNA by PCR of samples from cerebro spinal fluid) were . A PCR test that quantitates cytomegalovirus ( CMV ) DNA in human plasma or whole blood.