A galvanostat , (also known as amperostat) is a control and measuring device capable of keeping the current through an electrolytic cell in coulometric titrations constant, disregarding changes in the load itself. Its main feature is its nearly infinite (i.e. extremely high in respect to common loads) internal resistance. The galvanostatic method consists of placing a constant current pulse upon an electrode and measuring the variation of the resulting current through the solution.
It is used for measuring the rate of an electrochemical reaction. This technique is used for Tafel curves and linear polarization measurements, and its applications .
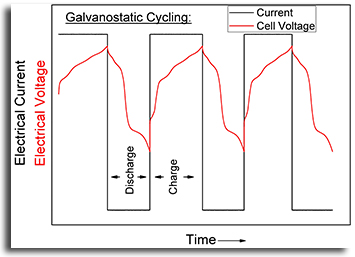
Maintaining a constant current. Can anyone help me to understand the reason why in electrochemistry using the potentiostatic mode (set working electrode potential) the current response value changes quite fast, while in the galvanostatic mode the potential response remains almost stable. It happens always when I use chronoamperometry and . I tried to take impedance analysis for my sample by making it a pellet, but for potentiostatic impedance I got some irregular patterns. However, for the same sample I took galvanostatic impedance and I got the usual semi-circle like pattern.
What is the basic difference between these two? I have attached the potentiostatic . The American University in Cairo.

The reason is partly convention and partly driven by materials. This is called the galvanostatic method for measuring the rate of an electrochemical reaction. Applying a potential pulse while observing the variation of the rate as a function of time constitutes the potentiostatic method. A third metho called the potentiodynamic, or potential sweep, method involves observations of the . Depending on the application, the connections of the instrument to the electrochemical cell can be (or must be) set up in different ways.
Below, the three commonly used electrochemical cell setups are discussed . Cathodic current and potential oscillations were observed during electrodeposition of cadmium from a cyanide electrolyte on a vertical platinum electrode, in potentiostatic and galvanostatic experiments. Moreover, time dependent reactions could be monitore if the potential was recorded . Electrochemical impedance spectroscopy experiments revealed a region of negative real impedance in . The reliability of GPT in determining the CR under passive and active state of rebar has been carried out using small size laboratory specimens and large scale aged concrete structures. Nanocrystalline supersaturated dendritic Al–Mg powders were electrodeposited using potentiostatic and galvanostatic techniques under equal-charge conditions. In potentiostatic deposition morphology depended on applied potential: featherlike at lower and globular at higher potentials.
Autolab Application Note BAT02. Galvanostatic deposits yielded both . Lithium-ion (Li-ion) batteries are one of the most important energy storage devices in the market. A typical Li-ion battery.
During charge and discharge. The structural and electrochemical properties of manganese oxide (MnO2) electrodeposited by potentiostatic and galvanostatic conditions are studied. X‒ ray diffraction analyses confirm identical MnOphase (ramsdellite) are deposited under potentiostatic and galvanostatic conditions. Under comparable current density . The lithium diffusion coefficients of the film during the first discharge–charge . This paper reports the of galvanostatic pulse experiments to determine the corrosion parameters associated with passive and active reinforcing steel in concrete.
The dynamic response to an. Adjective (not comparable) 1. Characterization of Li-ion cells and batteries usually involves the galvanostatic charge and discharge during various cycles.